Carbon Dioxide Monitoring in Neonatal Patients
CO2 Monitoring in Neonatal Patients
CO2 Monitoring in Neonatal Patients
 Carbon Dioxide and the Neonatal Brain
Carbon Dioxide and the Neonatal Brain
Brain protection is a high priority in preterm infants. Carbon dioxide (CO2 )is a critical monitoring parameter due to its impact on cerebral blood flow and the reduced ability of preterm infants to regulate that blood flow. Hypocarbia and hypercarbia, as well as large swings between the extremes, have been linked to intraventricular hemorrhage1, which happens in as many as 252-42%3 of neonates weighing less than 1500g at birth. To ensure that CO2 remains in a safe range and support better outcomes for NICU patients, CO2 levels must be measured and monitored closely.
CO2 and the Lungs
To manage the CO2, the main tool is your ventilation strategy. However, freely increasing the rate, volume, and pressure without careful consideration presents its own set of issues. The ventilation that is important to keep CO2 in a safe range for brain protection is the same ventilation that could damage the lungs. Mechanical ventilation and its duration have been linked to bronchopulmonary dysplasia, pulmonary hypertension, and neurodevelopmental impairment4. Volumes and pressure too great contribute to overdistension and volutrauma, while too little result in derecruitment and atelectasis. Continuous, accurate CO2 measurements help determine the ideal ventilator support to protect the brain and lungs.
How do we measure CO2 levels in NICU patients?
The gold standard for CO2 measurements is through arterial blood gases (ABG) or capillary heel sticks, commonly seen in the NICU. Blood gases have their advantages, as they are typically accurate and provide information about multiple parameters in one measurement. However, there is a hidden cost that goes along with each stick. Blood draws have been connected to blood loss, risk of infection, as well as increased pain and stimulation which can have long-lasting consequences6,7. There is also the time cost to consider as there is a lag time between taking the sample and receiving the result, not to mention the time spent gathering supplies and the clinician to draw the sample.
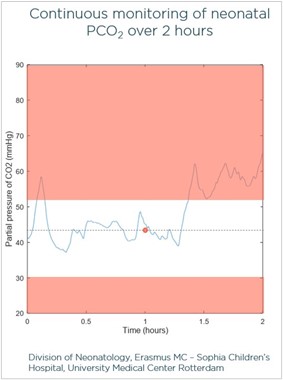 It is also important to realize that a blood gas is showing you information at one point in time. Take this example of a CO2 measurement in a neonatal patient. The blood gas taken at the one-hour mark showed that the patient’s CO2 was around 43 mmHg. However, the overlying tracing shows the transcutaneous CO2 trend throughout the two-hour monitoring period. Notice how the CO2 was higher before and after the blood gas, at some points reaching the point of hypercapnia. By relying on a blood gas measurement alone, clinicians may miss important changes and fluctuations in CO2.
It is also important to realize that a blood gas is showing you information at one point in time. Take this example of a CO2 measurement in a neonatal patient. The blood gas taken at the one-hour mark showed that the patient’s CO2 was around 43 mmHg. However, the overlying tracing shows the transcutaneous CO2 trend throughout the two-hour monitoring period. Notice how the CO2 was higher before and after the blood gas, at some points reaching the point of hypercapnia. By relying on a blood gas measurement alone, clinicians may miss important changes and fluctuations in CO2.
How can we measure CO2 continuously?
Transcutaneous CO2 (tcPCO2) monitoring provides an accurate estimation of arterial CO2 by measuring CO2 as it diffuses across the skin, allowing clinicians to monitor CO2 values continuously and noninvasively. Unlike end-tidal CO2, which presents feasibility and accuracy issues in the NICU population, tcPCO2 technology allows clinicians to monitor patients regardless of ventilatory support or lung compromise, from high-flow nasal cannula to high frequency oscillatory ventilation.
Overall, transcutaneous technology offers accurate, continuous, noninvasive CO2 values regardless of ventilation method or V/Q mismatch, all while supporting neuroprotective efforts to deliver clustered care, protect skin integrity, and reduce the frequency of blood draws.
References
- Hochwald O, Borenstein-Levin L, Dinur G, Jubran H, Ben-David S, Kugelman A. Continuous Noninvasive Carbon Dioxide Monitoring in Neonates: From Theory to Standard of Care. Pediatrics. 2019;144(1):e20183640. doi:10.1542/peds.2018-3640
- Database of Very Low Birth Weight Infants Born in 2012. Burlington, VT: Vermont Oxford Network, 2013. Nightingale Internet Reporting System, accessed April 4, 2014.
- Ahn SY, Shim SY, Sung IK. Intraventricular Hemorrhage and Post Hemorrhagic Hydrocephalus among Very-Low-Birth-Weight Infants in Korea. J Korean Med Sci. 2015;30 Suppl 1(Suppl 1):S52-S58. doi:10.3346/jkms.2015.30.S1.S52
- Choi et al. The Journal of Pediatrics, Volume 194, 34 – 39.e3
- Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia–effective and promising strategies for reducing transfusion. Semin Perinatol. 2012;36(4):232-243. doi:10.1053/j.semperi.2012.04.003
- Duerden et al. Early Procedural Pain Is Associated with Regionally-Specific Alterations in Thalamic Development in Preterm Neonates. J Neurosci. 2018;38(4):878-886. doi:10.1523/JNEUROSCI.0867-17.2017
- Brummelte et al. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71(3):385-396. doi:10.1002/ana.22267

