Overcoming the Limitations of End-Tidal CO2 Monitoring in the Sleep Lab
When sleep lab teams want to monitor ventilation, it’s important to understand the limitations of end-tidal CO2 monitoring and how it can affect their sleep lab patients. In this post we’ll be breaking down the basics of end-tidal CO2 monitoring and its clinical uses, and explore some of its limitations for a variety of patients in the sleep lab.
Overcoming the Limitations of End-Tidal CO2 Monitoring in the Sleep Lab
When sleep lab teams want to monitor ventilation, it’s important to understand the limitations of end-tidal CO2 monitoring and how it can affect their sleep lab patients. In this post we’ll be breaking down the basics of end-tidal CO2 monitoring and its clinical uses, and explore some of its limitations for a variety of patients in the sleep lab.
Overcoming the Limitations of End-Tidal CO2 Monitoring in the Sleep Lab
When sleep lab teams want to monitor ventilation, it’s important to understand the limitations of end-tidal CO2 monitoring and how it can affect their sleep lab patients. In this post we’ll be breaking down the basics of end-tidal CO2 monitoring and its clinical uses, and explore some of its limitations for a variety of patients in the sleep lab.
What is end-tidal CO2 monitoring and how is it used in the sleep lab?
End-tidal CO2 monitoring is a non-invasive method that measures the partial pressure of carbon dioxide at the end of each exhaled breath. The primary function of end-tidal CO2 monitoring in the sleep lab is to provide breath-to-breath measurement for the identification of apnea, as well as continuous monitoring of CO2. Being able to continuously monitor patients in the sleep lab helps registered polysomnographic technologists (RPSGTs) and other clinicians have better visibility into their patients’ ventilatory status.
The limitations of end-tidal CO2 monitoring in the sleep lab
While the continuous nature of end-tidal CO2 monitoring is an advantage, the technology faces challenges when it comes to measuring CO2 accurately in some scenarios, specifically in the patient population seen in the sleep lab:
- End-tidal often underestimates CO2 in patients with a ventilation/perfusion (V/Q) mismatch.
- For patients on NIV, end-tidal may misrepresent PaCO2 due to gas mixing and leakage.
- In split-night studies, end-tidal can struggle to provide congruent information. As ventilation methods change, so does end-tidal’s estimation of PaCO2.
- Patient compliance can be a challenge during sleep studies, especially in the pediatric population.
Here, we’ll dig a bit deeper into some of these challenges, and how they can be overcome.
Patients with V/Q mismatch

In patients with healthy lungs who breathe normally, etCO2 offers a reliable estimation of arterial CO2. However, in patients with V/Q mismatch, etCO2 is deficient. This is due to the fundamental, physiological nature of impaired gas exchange brought about by V/Q mismatch.
For a deeper discussion on CO2 monitoring and V/Q mismatch, explore our whitepaper.
CO2 in the breath does not represent the CO2 in the blood
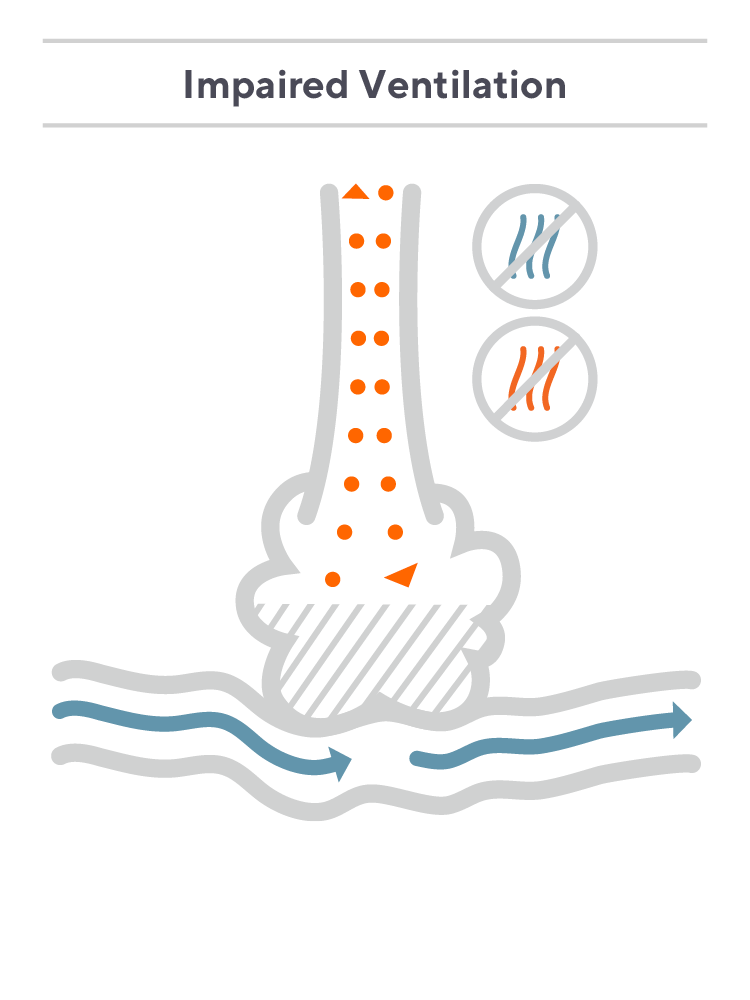
Consider a hypothetical patient with asthma who has a degree of V/Q mismatch due to impaired ventilation. In this case, some oxygen may flow in as the patient inhales, but due to restrictions in airflow, the majority of the oxygen is unable to reach the alveoli, and only some diffuses into the bloodstream. Largely, the patient is exhaling the O2 they just inhaled.
Meanwhile, the CO2 in the patient’s blood diffuses out into the lungs, but because airflow is restricted, an insignificant amount is exhaled. An end-tidal sensor will then measure a diluted sample of CO2 in their exhaled breath, which does not accurately represent their PaCO2.The patient’s etCO2 will be lower than their PaCO2 value as measured with a blood draw—in some cases significantly so.¹
Said simply: For patients with V/Q mismatch, the CO2 level in their breath does not match the CO2 level in their blood, significantly limiting end-tidal’s accuracy. Underestimating CO2 levels can lead to insufficient or delayed ventilatory patient management.
V/Q mismatch and end-tidal monitoring in patients with poor perfusion
EtCO2 monitoring is similarly challenged for patients with poor perfusion.
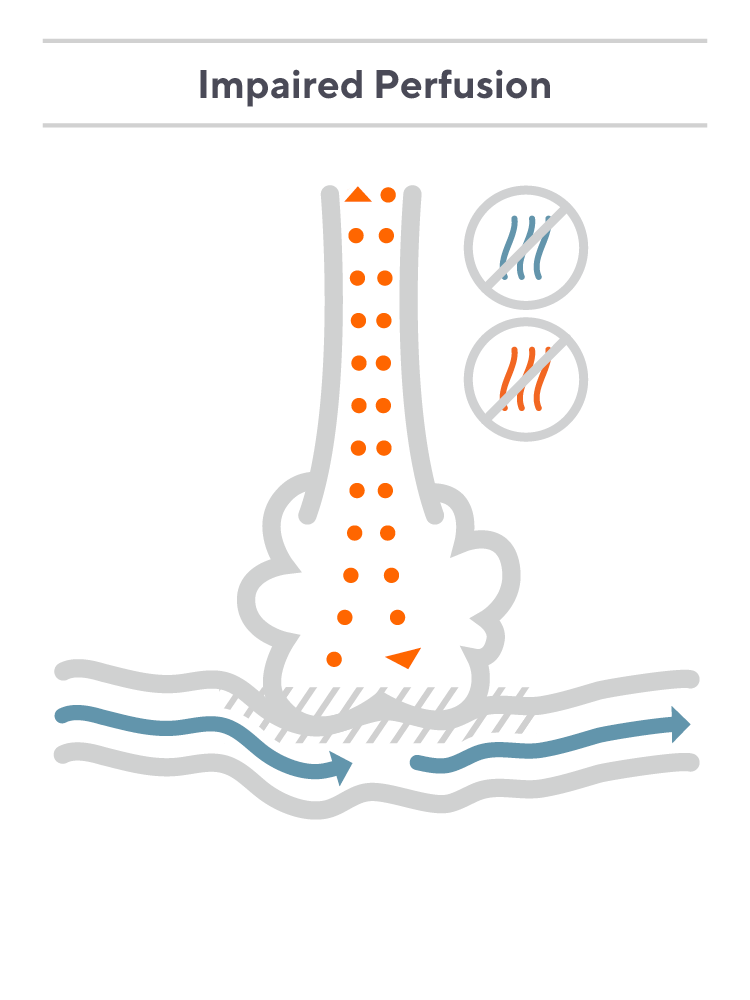
In a hypothetical patient who is ventilated using positive pressure ventilation, alveoli may become overdistended, compressing the pulmonary capillaries and limiting perfusion.
In this scenario, air travels through the airway to the alveoli, but due to the poor perfusion, there is minimal blood flow for the oxygen to diffuse into, and the oxygen is mostly exhaled. At the same time, CO2 doesn’t effectively diffuse into the lungs and is retained in the bloodstream, increasing the patient’s PaCO2.
For a patient like this, the CO2 in their breath will not match the CO2 in their blood, and the end-tidal reading will underestimate PaCO2. Again, underestimating a patient’s CO2 level can lead to insufficient management of their ventilatory status.
Accurate, continuous CO2 monitoring for patients with V/Q mismatch
In the sleep lab, it is common to observe patients with varying degrees of VQ mismatch, highlighting the significance of addressing the challenges related to end-tidal monitoring. This includes individuals with asthma, obstructive sleep apnea, COPD, chronic bronchitis, pneumonia, pulmonary hypertension, collapsed alveoli, pulmonary embolism, and more.
The challenges of end-tidal CO2 monitoring in the sleep lab can be addressed with the use of transcutaneous monitoring. Transcutaneous CO2 monitoring utilizes a noninvasive sensor that gently warms the skin at the measurement site to 41 C°, which encourages blood flow and diffusion of gases through the skin. The CO2 then passes through a permeable membrane on the sensor face and into the specially formulated electrolyte, where a measurable reaction takes place. Proprietary algorithms then translate the data into an estimate of PaCO2.
Because transcutaneous monitoring measures CO2 through the skin rather than the breath, alveolar perfusion and ventilation are irrelevant to the accuracy of the measurement. By sampling in capillary beds, tcPCO2 provides an accurate ¹ ³ 4 estimation of PaCO2 and largely avoids the issues of V/Q mismatch experienced by etCO2.
As one pair of researchers stated in Critical Care Medicine,
“End-tidal CO2 monitoring has been shown to accurately estimate ventilation in patients with normal pulmonary function. However, in the critically ill, where pulmonary function is often abnormal, this accuracy becomes limited because intrapulmonary shunting and increased alveolar dead space result in a consistent underestimation of PaCO2 content. Conversely, the accuracy of tcPCO2 monitoring is independent of pulmonary status and may bypass some of the problems inherent to etCO2 monitoring.”²

Gas Mixing
Gas mixing presents a similar concern as V/Q mismatch when it comes to end-tidal monitoring. It is crucial to keep in mind that end-tidal sensors detect a breath sample, meaning that any alteration in airflow over the sensor will directly affect its measurement, whether coming from the patient’s exhale or another source.
Complicated and diverse airflow can be introduced in patients who are on respiratory support devices. Consequently, the end-tidal measurement can comprise a mixture of the patient’s breath and any flow from the respiratory assist device, rather than a pure sample of a patient’s exhaled breath.
For instance, patients using CPAP or BiPAP may face challenges in accurately measuring arterial CO2 levels through end-tidal readings, even when their V/Q ratio is normal. This is because the gas sample collected within the mask comprises not only the patient’s exhaled breath but also the flow from the CPAP or BiPAP device.
It is also important to consider that when placing a mask over the measurement cannula, the cannula might not allow for a complete seal. This can result in gas leakage both into and out of the mask, which can further disturb the air that is being sampled during end-tidal monitoring.
How does transcutaneous monitoring overcome the challenge of gas mixing?
Transcutaneous monitoring offers a distinct advantage over end-tidal monitoring by eliminating the reliance on airway function or the possibility of mask interface. Transcutaneous sensors are placed on the skin and measure CO2 through the capillary beds. This approach, entirely independent of ventilation modality, addresses concerns related to gas mixing and dilution, allowing for accurate estimation of a patient’s arterial CO2 levels.
Monitoring CO2 during split-night sleep studies
During split-night sleep studies, respiratory support (typically CPAP) is often introduced midway through the patient’s sleep cycle. This aims to assess the disparity in their respiratory condition with and without respiratory assistance.
It is possible to obtain an accurate reading of CO2 with end-tidal monitoring during the unmasked portion of a split-night study, as long as the cannula remains in place and the patient maintains regular breathing. However, it is important to note that this may not hold true for patients with certain breathing dysfunctions, such as obstructive sleep apnea (OSA), which can contribute to V/Q mismatch.
If end-tidal struggles to maintain accuracy due to gas mixing resulting from added support, the supported portion of the sleep study may fail to offer insights into the effectiveness of the support on the patient’s ventilatory status.
Overall, the unreliability of end-tidal CO2 monitoring during a split-night study may result in more questions than answers concerning a patient’s ventilatory status:
- If CO2 has dropped after a mask has been placed, is that because of improved ventilation, or is the end-tidal sample diluted by gas mixing?
- If the patient begins snoring and displaying other symptoms of OSA, are their CO2 levels abnormal or are the end-tidal reading impaired due to V/Q mismatch?
- If a patient is restless during the night or noncompliant, is the recorded data accurate or was their cannula accidentally removed?
How can transcutaneous monitoring overcome the challenges with split-night studies?
Because transcutaneous CO2 monitoring measures through the skin, its accuracy is not dependent on consistent ventilation of the patient, and whether or not they are masked for a portion or the entirety of a night does not change the accuracy of transcutaneous CO2 measurements.
Since transcutaneous monitoring is not relying on cannula placement for its measurement, noncompliant patients who may find wearing cannulas difficult also do not create accuracy issues with transcutaneous monitoring.
Additionally, Sentec transcutaneous CO2 monitoring systems have built-in features to help sleep technologists monitor CO2 throughout the night with confidence, including:
- Smart Cal-Mem | Allows for the disconnection and reconnection of the sensor from the monitor for up to 30-minutes without the need for recalibration. This allows patients to get up to use the bathroom without the need for recalibration or reapplication of the sensor.
Learn more about the clinical impact of transcutaneous CO2 monitoring in the sleep lab
Sentec transcutaneous technology overcomes limits of pulse oximetry, arterial blood draws, and capnography with continuous CO2 monitoring that combines ease of use and patient comfort. It provides accurate values regardless of ventilation method or V/Q mismatch, and is designed with features with the sleep care setting in mind.
References:
- Cox, P., et al. Noninvasive Monitoring of PaCO2 during One-Lung Ventilation and Minimal Access Surgery in Adults: End-Tidal versus Transcutaneous Techniques. Journal of Minimal Access Surgery. 2007.
- Berkenbosch, J.W., Tobias, J. Transcutaneous Carbon Dioxide Monitoring During High-Frequency Oscillatory Ventilation in Infants and Children. Critical Care Medicine. 2002.
- Don, D., et al. Transcutaneous CO2 Monitoring in Children Undergoing Tonsillectomy for Sleep Disordered Breathing. The Laryngoscope. 2020.
- Schwarz, S.B., et al. Continuous Non-Invasive PCO2 Monitoring in Weaning Patients: Transcutaneous is Advantageous Over End-Tidal PCO2. Respirology. 2017.


